
A gradual gradation in the physical and chemical properties of elements is clearly observed.ģ. There is a good correlation between the properties of the element and its position in the periodic table.Ģ. All the f-block elements are also known as Inner Transltion elements.Īdvantages of this type of classification :ġ. The elements of second row are called actinides and in these elements the valence electron enters into 5f orbital. The elements of first row are called lanthanides and in these elements the valence electron enters into 4f orbital. The f-block elements are arranged separately at the bottom in two rows.
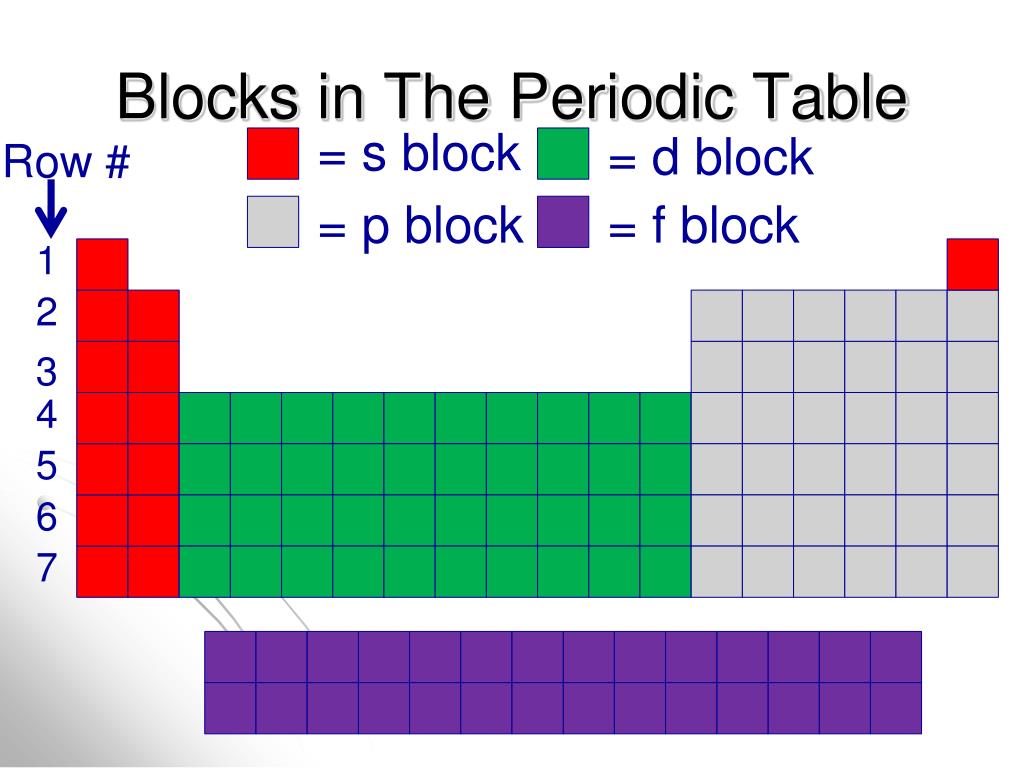
Their general outer configuration is ( n − 2 ) f 1 − 14 ( n − 1 ) d 0 − 1 n s 2. These elements are also known as Transition elements.į-Block : The elements in which the differentiating electron enters into f-orbital are called f-block elements. The general outer configuration of d-block elements is ( n − 1 ) d 1 − 10 n s 1 − 2. They are IIIB, IVB, VB, VIB, VIIB, VIII, IB, IIB. The valence electron configuration of the elements of these groups varies from n s 2 n p 1 to n s 2 n p 6ĭ-Block : The elements in which the differentiating electron enters into d- sub-level are called d-block elements. P-Block : The elements in which the differentiating electron enters the p- sub-level are called p-block elements.

The valence electron configuration of elements of IA group is n s 1 and that of IIA is n s 2 S-block : The elements, in which the differentiating electron enters the s- sub-level are called s-block elements. Depending upon the entering of differentiating electrn into the atomic orbitals, the elements of long form of periodic table are divided into four blocks.


 0 kommentar(er)
0 kommentar(er)
